Brillouin microscopy monitors rapid responses in subcellular compartments
-
Abstract:
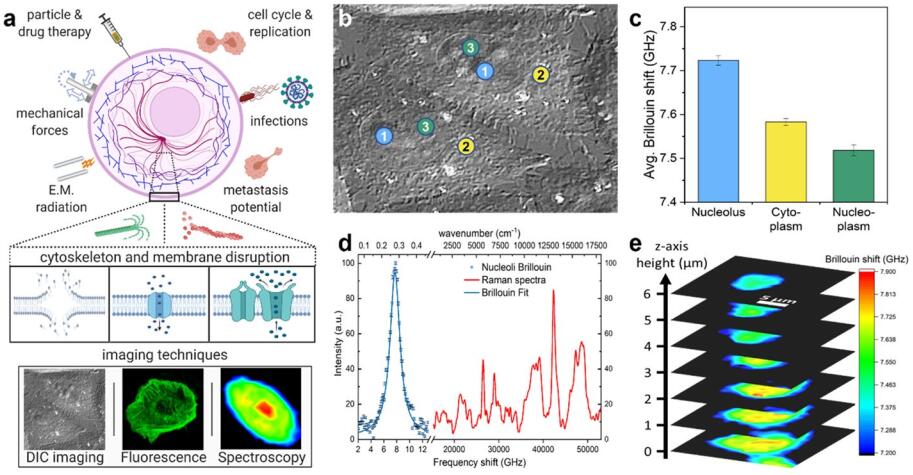
Measurements and imaging of the mechanical response of biological cells are critical for understanding the mechanisms of many diseases, and for fundamental studies of energy, signal and force transduction. The recent emergence of Brillouin microscopy as a powerful non-contact, label-free way to non-invasively and non-destructively assess local viscoelastic properties provides an opportunity to expand the scope of biomechanical research to the sub-cellular level. Brillouin spectroscopy has recently been validated through static measurements of cell viscoelastic properties, however, fast (sub-second) measurements of sub-cellular cytomechanical changes have yet to be reported. In this report, we utilize a custom multimodal spectroscopy system to monitor for the very first time the rapid viscoelastic response of cells and subcellular structures to a short-duration electrical impulse. The cytomechanical response of three subcellular structures - cytoplasm, nucleoplasm, and nucleoli - were monitored, showing distinct mechanical changes despite an identical stimulus. Through this pioneering transformative study, we demonstrate the capability of Brillouin spectroscopy to measure rapid, real-time biomechanical changes within distinct subcellular compartments. Our results support the promising future of Brillouin spectroscopy within the broad scope of cellular biomechanics.
-
Key words:
- Engineering" /
- " data-track="click" data-track-action="view keyword" data-track-label="link">Microscopy /
- Engineering" /
- " data-track="click" data-track-action="view keyword" data-track-label="link">Imaging /
- Engineering" /
- " data-track="click" data-track-action="view keyword" data-track-label="link">Brillouin scattering /
- Engineering" /
- " data-track="click" data-track-action="view keyword" data-track-label="link">Raman scattering /
- Engineering" /
- " data-track="click" data-track-action="view keyword" data-track-label="link">Fluorescence
-
[1] Jonietz E. Mechanics: the forces of cancer. Nature. 2012;491:S56–7. [2] Panciera T, et al. Reprogramming normal cells into tumour precursors requires ECM stiffness and oncogene-mediated changes of cell mechanical properties. Nat Mater. 2020;19:797–806. [3] Yuan S, Norgard RJ, Stanger BZ. Cellular plasticity in cancer. Cancer Discov. 2019;9:837–51. [4] Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat Nanotech. 2007;2:780–3. [5] Han YL, et al. Cell swelling, softening and invasion in a three-dimensional breast cancer model. Nat Phys. 2020;16:101–8. [6] Lam V, Bigley T, Terhune SS, Wakatsuki T. A method for quantifying mechanical properties of tissue following viral infection. PLoS One. 2012;7:e42197. [7] Kräter M, et al. Alterations in cell mechanics by actin cytoskeletal changes correlate with strain-specific rubella virus phenotypes for cell migration and induction of apoptosis. Cells. 2018;7:136. [8] Lampi MC, Reinhart-King CA. Targeting extracellular matrix stiffness to attenuate disease: from molecular mechanisms to clinical trials. Sci Transl Med. 2018;10:eaao0475. [9] Phillip JM, Aifuwa I, Walston J, Wirtz D. The mechanobiology of aging. Annu Rev Biomed Eng. 2015;17:113–41. [10] Liu B, McNally S, Kilpatrick JI, Jarvis SP, O’Brien CJ. Aging and ocular tissue stiffness in glaucoma. Surv Ophthalmol. 2018;63:56–74. [11] Overby DR, et al. Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. Proc Natl Acad Sci U S A. 2014;111:13876–81. [12] Xu W, et al. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE. 2012;7: e46609. [13] Raudenska M, et al. Cisplatin enhances cell stiffness and decreases invasiveness rate in prostate cancer cells by actin accumulation. Sci Rep. 2019;9:1660. [14] Huang J, et al. Extracellular matrix and its therapeutic potential for cancer treatment. Sig Transduct Target Ther. 2021;6:1–24. [15] Cecelja M, Shanahan CM. Targeting cell stiffness. Circul Res. 2021;128:769–71. [16] Paluch E, Heisenberg CP. Biology and physics of cell shape changes in development. Curr Biol. 2009;19:R790-799. [17] Handorf AM, Zhou Y, Halanski MA, Li W-J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis. 2015;11:1–15. [18] Nematbakhsh Y, Lim CT. Cell biomechanics and its applications in human disease diagnosis. Acta Mech Sin. 2015;31:268–73. [19] Quan F-S, Kim KS. Medical applications of the intrinsic mechanical properties of single cells. Acta Biochim Biophys Sin. 2016;48:865–71. [20] Guimarães CF, Gasperini L, Marques AP, Reis RL. The stiffness of living tissues and its implications for tissue engineering. Nat Reviews Mater. 2020;5:351–70. [21] Essmann CL, et al. Mechanical properties measured by atomic force microscopy define health biomarkers in ageing C. Elegans. Nat Commun. 2020;11:1043. [22] Guillou L, Babataheri A, Puech P-H, Barakat AI, Husson J. Dynamic monitoring of cell mechanical properties using profile microindentation. Sci Rep. 2016;6: 21529. [23] Zhang Y, et al. Interfacing 3D magnetic twisting cytometry with confocal fluorescence microscopy to image force responses in living cells. Nat Protoc. 2017;12:1437–50. [24] Herráez-Aguilar D, et al. Multiple particle tracking analysis in isolated nuclei reveals the mechanical phenotype of leukemia cells. Sci Rep. 2020;10:6707. [25] Guck J, et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J. 2005;88:3689–98. [26] Wang S, Larin KV. Optical coherence elastography for tissue characterization: a review. J Biophoton. 2015;8:279–302. [27] Larin KV, Sampson DD. Optical coherence elastography – OCT at work in tissue biomechanics. Biomed Opt Express. 2017;8:1172–202. [28] Meng Z, Traverso AJ, Ballmann CW, Troyanova-Wood MA, Yakovlev V. V. Seeing cells in a new light: a renaissance of Brillouin spectroscopy. Adv Opt Photon. 2016;8:300–27. [29] Prevedel R, Diz-Muñoz A, Ruocco G, Antonacci G. Brillouin microscopy: an emerging tool for mechanobiology. Nat Methods. 2019;16:969–77. [30] Palombo F, Fioretto D. Brillouin light scattering: applications in biomedical sciences. Chem Rev. 2019;119:7833–47. [31] Scarcelli G, et al. Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy. Nat Methods. 2015;12:1132–4. [32] Pakhomov AG, et al. Disassembly of actin structures by nanosecond pulsed electric field is a downstream effect of cell swelling. Bioelectrochemistry. 2014;100:88–95. [33] Carr L, et al. Calcium-independent disruption of microtubule dynamics by nanosecond pulsed electric fields in U87 human glioblastoma cells. Sci Rep. 2017;7: 41267. [34] Meng Z, Bustamante Lopez SC, Meissner KE, Yakovlev VV. Subcellular measurements of mechanical and chemical properties using dual Raman-Brillouin microspectroscopy. J Biophotonics. 2016;9:201–7. [35] Fiore A, Zhang J, Shao P, Yun SH, Scarcelli G. High-extinction VIPA-based Brillouin spectroscopy of turbid biological media. Appl Phys Lett. 2016;108:1–9. [36] Antonacci G, Braakman S. Biomechanics of subcellular structures by non-invasive Brillouin microscopy. Sci Rep. 2016;6:37217. [37] Mattana S, et al. Non-contact mechanical and chemical analysis of single living cells by microspectroscopic techniques. Light: Sci Appl. 2018;7:17139–17139. [38] Troyanova-Wood M, Meng Z, Yakovlev VV. Differentiating melanoma and healthy tissues based on elasticity-specific Brillouin microspectroscopy. Biomed Opt Express BOE. 2019;10:1774–81. [39] Margueritat J, et al. High-frequency mechanical properties of tumors measured by Brillouin light scattering. Phys Rev Lett. 2019;122:018101. [40] Zhang J, et al. Nuclear mechanics within intact cells is regulated by cytoskeletal network and internal nanostructures. Small. 2020;16:1907688. [41] Batista Napotnik T, Reberšek M, Vernier PT, Mali B, Miklavčič D. Effects of high voltage nanosecond electric pulses on eukaryotic cells (in vitro): a systematic review. Bioelectrochemistry. 2016;110:1–12. [42] Kotnik T, Rems L, Tarek M, Miklavčič D. Membrane electroporation and electropermeabilization: mechanisms and models. Annu Rev Biophys. 2019;48:63–91. [43] Antonacci G, et al. Recent progress and current opinions in Brillouin microscopy for life science applications. Biophys Rev. 2020;12:615–24. [44] Thompson GL, Roth C, Tolstykh G, Kuipers M, Ibey BL. Disruption of the actin cortex contributes to susceptibility of mammalian cells to nanosecond pulsed electric fields. Bioelectromagnetics. 2014;35:262–72. [45] Tolstykh GP, Thompson GL, Beier HT, Steelman ZA, Ibey BL. nsPEF-induced PIP2 depletion, PLC activity and actin cytoskeletal cortex remodeling are responsible for post-exposure cellular swelling and blebbing. Biochem Biophys Rep. 2017;9:36–41. [46] Thompson GL, Roth CC, Dalzell DR, Kuipers M, Ibey BL. Calcium influx affects intracellular transport and membrane repair following nanosecond pulsed electric field exposure. J Biomed Opt. 2014;19:055005. [47] Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–14. [48] O’Brien ET, Salmon ED, Erickson HP. How calcium causes microtubule depolymerization. Cell Motil. 1997;36:125–35. [49] Steinkühler J, Sezgin E, Urbančič I, Eggeling C, Dimova R. Mechanical properties of plasma membrane vesicles correlate with lipid order, viscosity and cell density. Commun Biol. 2019;2:337. [50] Guck J. Some thoughts on the future of cell mechanics. Biophys Rev. 2019;11:667–70. [51] Ibey BL, Xiao S, Schoenbach KH, Murphy MR, Pakhomov AG. Plasma membrane permeabilization by 60- and 600-ns electric pulses is determined by the absorbed dose. Bioelectromagnetics. 2009;30:92–9. [52] Steelman ZA, Tolstykh GP, Beier HT, Ibey BL. Cellular response to high pulse repetition rate nanosecond pulses varies with fluorescent marker identity. Biochem Biophys Res Commun. 2016;478:1261–7. [53] Steelman ZA, et al. Quantitative phase microscopy monitors subcellular dynamics in single cells exposed to nanosecond pulsed electric fields. J Biophotonics. 2021;14: e202100125. https://doi.org/10.1002/jbio.202100125. [54] Nesin OM, Pakhomova ON, Xiao S, Pakhomov AG. Manipulation of cell volume and membrane pore comparison following single cell permeabilization with 60- and 600-ns electric pulses. Biochim et Biophys Acta (BBA) - Biomembr. 2011;1808:792–801. [55] Breton M, Mir LM. Microsecond and nanosecond electric pulses in cancer treatments. Bioelectromagnetics. 2012;33:106. [56] Xiao D, et al. Irreversible electroporation and apoptosis in human liver cancer cells induced by nanosecond electric pulses. Bioelectromagnetics. 2013;34:512–20. [57] Rols M-P, et al. In vivo electrically mediated protein and gene transfer in murine melanoma. Nat Biotechnol. 1998;16:168–71. [58] Saldaña G, Álvarez I, Condón S, Raso J. Microbiological aspects related to the feasibility of PEF technology for food pasteurization. Crit Rev Food Sci Nutr. 2014;54:1415–26. [59] Caponi S, Fioretto D, Mattarelli M. On the actual spatial resolution of Brillouin imaging. Opt Lett OL. 2020;45:1063–6. [60] Antonacci G, Foreman MR, Paterson C, Török P. Spectral broadening in Brillouin imaging. Appl Phys Lett. 2013;103:5–8. [61] Zhang J, Scarcelli G. Mapping mechanical properties of biological materials via an add-on Brillouin module to confocal microscopes. Nat Protoc. 2021;16:1251–75. [62] Vincelette RL, et al. Thresholds for phosphatidylserine externalization in Chinese hamster ovarian cells following exposure to nanosecond pulsed electrical fields (nsPEF). PLoS ONE. 2013;8:e63122. [63] Nikolić M, Scarcelli G. Long-term Brillouin imaging of live cells with reduced absorption-mediated damage at 660nm wavelength. Biomed Opt Express BOE. 2019;10:1567–80. [64] Alisafaei F, Jokhun DS, Shivashankar GV, Shenoy VB. Regulation of nuclear architecture, mechanics, and nucleocytoplasmic shuttling of epigenetic factors by cell geometric constraints. Proc Natl Acad Sci USA. 2019;116:13200–9. [65] Steelman ZA, et al. Comprehensive single-shot biophysical cytometry using simultaneous quantitative phase imaging and Brillouin spectroscopy. Sci Rep. 2022;12:18285. [66] Troyanova-Wood M, Gobbell C, Meng Z, Gashev AA, Yakovlev VV. Optical assessment of changes in mechanical and chemical properties of adipose tissue in diet-induced obese rats. J Biophotonics. 2017;9:1–10. [67] Traverso AJ, et al. Dual Raman-Brillouin microscope for chemical and mechanical characterization and imaging. Anal Chem. 2015;87:7519–23. [68] Steelman Z, Meng Z, Traverso AJ, Yakovlev VV. Brillouin spectroscopy as a new method of screening for increased CSF total protein during bacterial meningitis. J Biophotonics. 2015;8:408–14. [69] Coker Z, et al. Assessing performance of modern Brillouin spectrometers. Opt Express. 2018;26:2400. [70] Meng Z, Yakovlev VV. Optimizing signal collection efficiency of the VIPA-based Brillouin spectrometer. J Innov Opt Health Sci. 2014;08:1550021. [71] Roth CC, et al. Characterization of pressure transients generated by nanosecond electrical pulse (nsEP) exposure. Sci Rep. 2015;5: 15063. [72] Lieber CA, Mahadevan-Jansen A. Automated method for subtraction of fluorescence from biological Raman spectra. Appl Spectrosc. 2003;57:1363–7. -
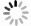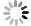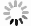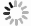








 下载:
下载: